Summary
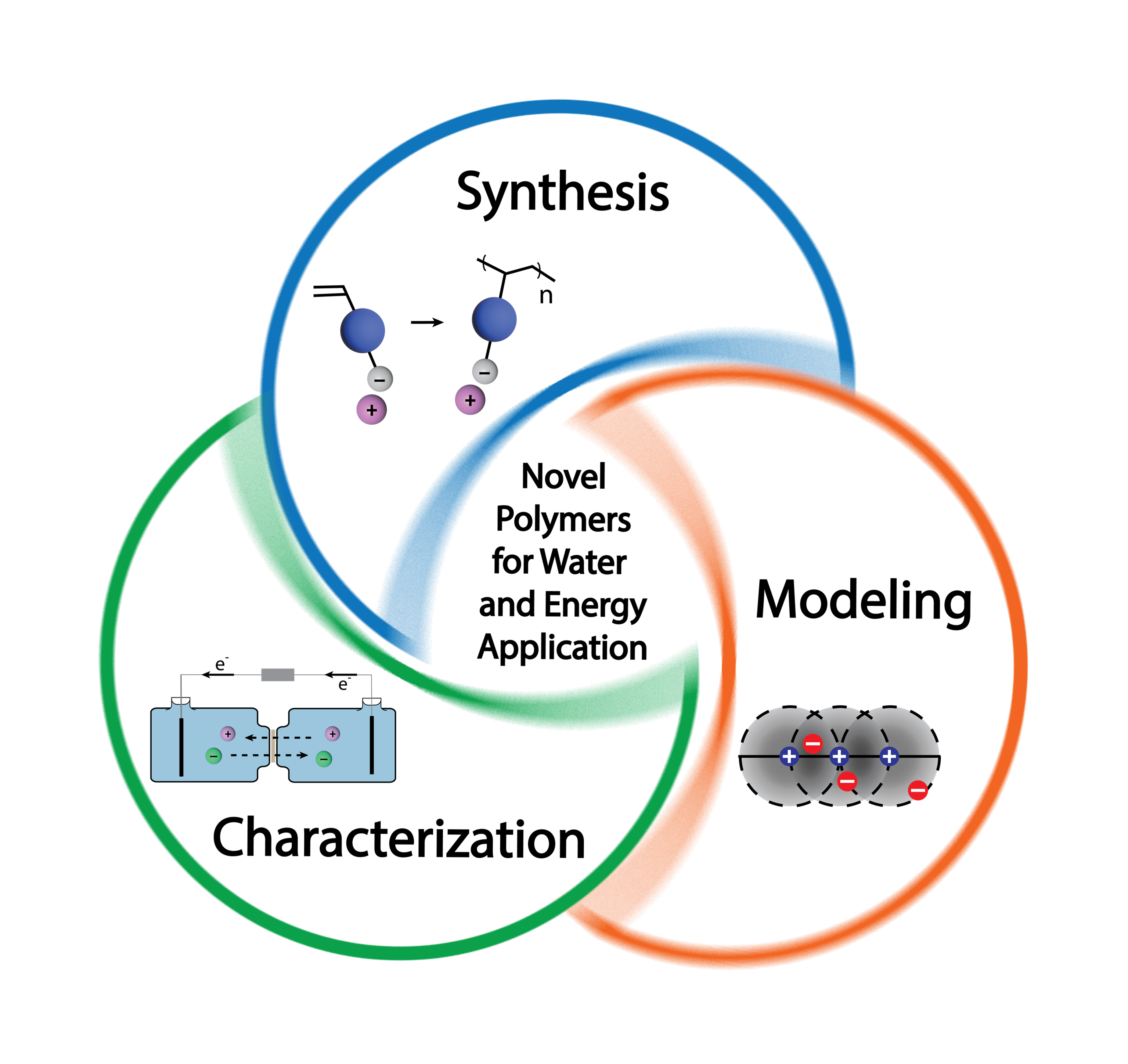 Securing adequate supplies of energy and water at affordable costs and in a sustainable manner is an enormous challenge facing humanity. Due to the interconnected relationship between these two critical resources, a shortcoming in one resource could negatively impact the availability of the other resource. Membrane-based technologies (e.g., reverse osmosis, electrodialysis, fuel cells, flow batteries) will play a key role in addressing our water and energy needs due to their efficiency, simplicity, and small footprint. The success of such technologies hinges on developing new materials with improved functionality. However, despite a longstanding academic and industrial interest in this area, significant fundamental and practical challenges remain. The overarching goal of the Kamcev research group is to develop next-generation polymeric materials (e.g., membranes and adsorbents) that will improve the efficiencies of existing technologies and enable the implementation of emerging technologies for water/energy applications. We implement an integrative approach based on materials synthesis, advanced characterization, and modeling to develop structure/property guidelines for the rational design of high-performance polymeric materials. We synthesize polymers with precisely controlled structures, characterize their physicochemical and transport properties, evaluate their performance in real systems, and, when appropriate, use models to establish connections between molecular structure and performance. Several of our ongoing research programs are summarized below.
Securing adequate supplies of energy and water at affordable costs and in a sustainable manner is an enormous challenge facing humanity. Due to the interconnected relationship between these two critical resources, a shortcoming in one resource could negatively impact the availability of the other resource. Membrane-based technologies (e.g., reverse osmosis, electrodialysis, fuel cells, flow batteries) will play a key role in addressing our water and energy needs due to their efficiency, simplicity, and small footprint. The success of such technologies hinges on developing new materials with improved functionality. However, despite a longstanding academic and industrial interest in this area, significant fundamental and practical challenges remain. The overarching goal of the Kamcev research group is to develop next-generation polymeric materials (e.g., membranes and adsorbents) that will improve the efficiencies of existing technologies and enable the implementation of emerging technologies for water/energy applications. We implement an integrative approach based on materials synthesis, advanced characterization, and modeling to develop structure/property guidelines for the rational design of high-performance polymeric materials. We synthesize polymers with precisely controlled structures, characterize their physicochemical and transport properties, evaluate their performance in real systems, and, when appropriate, use models to establish connections between molecular structure and performance. Several of our ongoing research programs are summarized below.
Fundamental studies of solute transport in charged polymer membranes
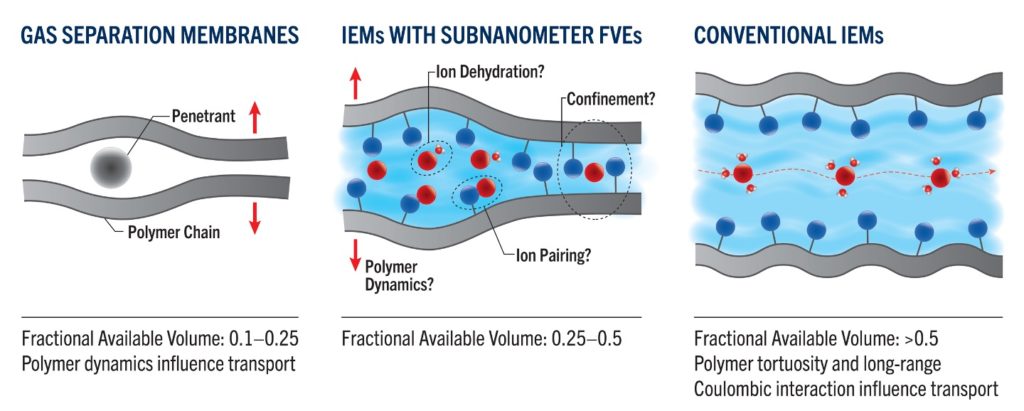
Rational design of new membranes with desired functionality would be accelerated by improved fundamental understanding of the connection between polymer structure and transport properties. One thrust of this program is to establish fundamental understanding of ion transport in highly charged polymer membranes with subnanometer free volume elements (FVEs). The properties of this unique and largely unexplored class of membranes are situated in a transition region between those of gas separation membranes, in which polymer backbone dynamics influence small molecule transport, and highly swollen charged membranes, in which tortuosity and long-range Coulombic interactions are most important. Better understanding of such materials could enable single species selectivity due to several effects that manifest in subnanometer pores, e.g., confinement and partial ion dehydration, as well as the influence of polymer backbone dynamics. Another thrust of this program is to establish fundamental understanding of the influence of charge delocalization on the transport properties of charged membranes for aqueous ion separation applications. By better understanding these largely unexplored topics, we will develop new tools for designing charged polymer membranes with improvements in both selectivity and throughput for applications that involve highly impaired waters, such as brine management and produced water treatment.
Novel adsorbents and mixed-matrix membranes for precision separation applications
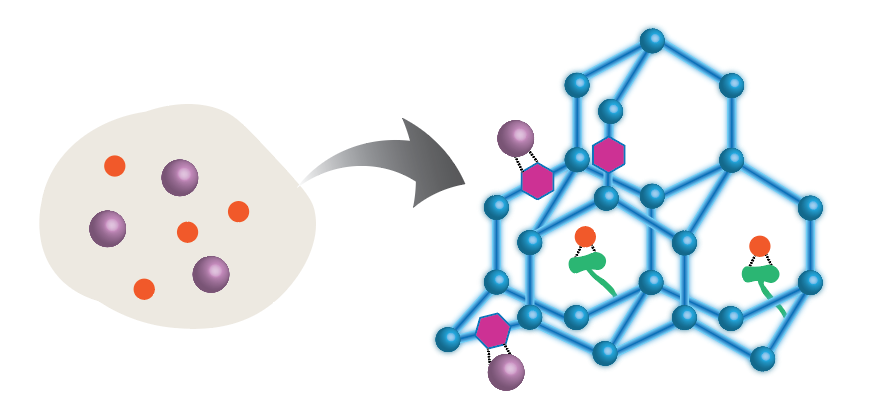 Current commercial membranes and adsorbents often exhibit inadequate selectivity for separation applications, which necessitates additional energy-intensive treatment steps to achieve desired separation targets. For example, state-of-the-art reverse osmosis membranes exhibit inadequate rejection of small, neutral solutes (e.g., boron, 1,4-dioxane, etc.), and charged membranes are often unable to discriminate between ions of similar charge (e.g., Li+ vs. Na+). Recent process modeling studies have demonstrated that improvements in membrane selectivity, rather than permeability, could lead to significantly higher efficiencies of separation technologies. There has been enormous interest over the past decade in designing microporous materials with precisely controlled chemical and physical structures, particularly for separation applications. These classes of materials, which include metal organic frameworks, covalent organic frameworks, and porous aromatic frameworks, have the potential to significantly surpass the separation performance of conventional polymeric materials. One thrust of this program is to design novel microporous materials with exceptional selectivity for target solutes (e.g., boron, lithium, etc.). We will accomplish this by synthetically controlling the physical structure (e.g., pore size, shape, and distribution) as well as the chemical structure (e.g., pore wall functionality) of the materials. Another thrust of this program is to combine the microporous materials with conventional polymers to form multi-functional mixed-matrix membranes that synergistically combine the benefits of highly selective adsorbents and highly processable polymers.
Current commercial membranes and adsorbents often exhibit inadequate selectivity for separation applications, which necessitates additional energy-intensive treatment steps to achieve desired separation targets. For example, state-of-the-art reverse osmosis membranes exhibit inadequate rejection of small, neutral solutes (e.g., boron, 1,4-dioxane, etc.), and charged membranes are often unable to discriminate between ions of similar charge (e.g., Li+ vs. Na+). Recent process modeling studies have demonstrated that improvements in membrane selectivity, rather than permeability, could lead to significantly higher efficiencies of separation technologies. There has been enormous interest over the past decade in designing microporous materials with precisely controlled chemical and physical structures, particularly for separation applications. These classes of materials, which include metal organic frameworks, covalent organic frameworks, and porous aromatic frameworks, have the potential to significantly surpass the separation performance of conventional polymeric materials. One thrust of this program is to design novel microporous materials with exceptional selectivity for target solutes (e.g., boron, lithium, etc.). We will accomplish this by synthetically controlling the physical structure (e.g., pore size, shape, and distribution) as well as the chemical structure (e.g., pore wall functionality) of the materials. Another thrust of this program is to combine the microporous materials with conventional polymers to form multi-functional mixed-matrix membranes that synergistically combine the benefits of highly selective adsorbents and highly processable polymers.
Membranes for electrochemical technologies for water treatment and energy generation/storage
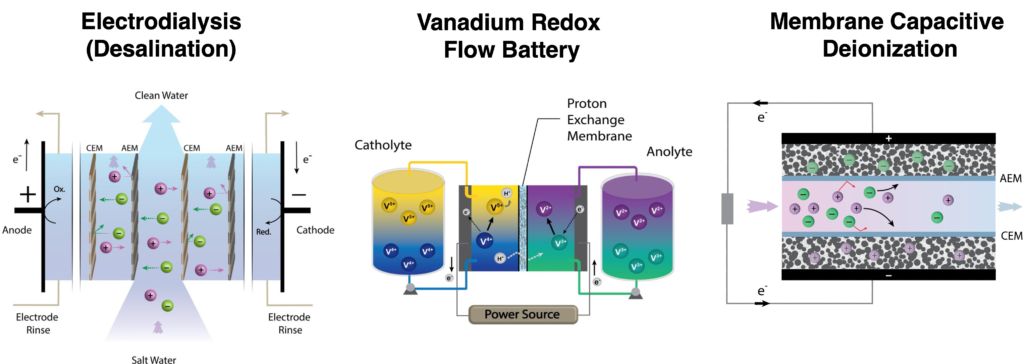
Membrane-based electrochemical technologies, such as electrodialysis, redox flow batteries, membrane capacitive deionization, fuel cells, and electrochemical CO2 reduction, will play a key role in meeting our future demands for water and energy. All of these technologies require membranes with different transport properties. For example, membranes must separate H+ from V2+, V3+, V4+, and V5+ in all-vanadium redox flow batteries; Na+ from Cl– in electrodialysis; or H+ from methanol in direct methanol fuel cells. Currently, there are a limited number of commercially available membranes that were developed and optimized for a small subset of these technologies (e.g., electrodialysis and fuel cells). New membranes with transport properties specifically designed for a given application are needed to accelerate adaptation of emerging environmental and energy technologies. The overarching objective of this program is to establish connections between membrane structure/properties and overall performance of electrochemical technologies (e.g., charge efficiency, energy consumption, stability, etc.), which will ultimately facilitate the design of new membranes for these technologies. Ongoing projects include studies on redox flow batteries (in collaboration with Prof. Nirala Singh), membrane capacitive deionization, and electrodialysis for brine concentration (in collaboration with SUEZ Water). We are also collaborating with Prof. Menachem Elimelech (Yale) to develop a novel bipolar membrane-assisted electrosorption technology for efficient boron removal from water.